The main metal alloys used in the mechanical field are iron, aluminium and copper alloys, thanks to their mechanical characteristics and their availability, cheapness and easy workability. The evolution of the market and technologies has inevitably led to research and demand for ever better characteristics, also with regard to surface characteristics. The three alloys mentioned often have insufficient surface characteristics, such as corrosion resistance or wear resistance. It is therefore frequently necessary to increase certain surface characteristics by applying coatings that can meet various technical and/or aesthetic requirements.
Electroless nickel plating is the most suitable coating for precision engineering parts as it combines several characteristics, such as uniform thickness, high hardness, wear resistance and excellent corrosion resistance.
The electroless nickel plating process is carried out by immersion in a nickel solution using a self-catalytic reaction process, without the use of electricity. A nickel-phosphorus alloy is deposited on the surface of the part to be treated by means of the oxidation-reduction reaction between the Ni++ cation from the nickel sulphate and the H2PO2- anion from the sodium hypophosphite (reducing agent).
This process produces a coating with uniform and calibrated thicknesses on all surfaces, including internal surfaces and complex geometries. This allows tight tolerances to be met without the need to insulate or rework tolerated surfaces. Moreover, the full coating of the workpiece allows to protect all internal surfaces such as cylinders, channels, fittings and distributors of air, water, steam or medical gases from corrosion and wear.
In galvanic (also known as electrolytic) processes, such as chromium or electrolytic nickel plating, electric current is used for the deposition of the metallic coating, which inevitably leads to increased thickness on the edges and difficulty in coating the internal areas.
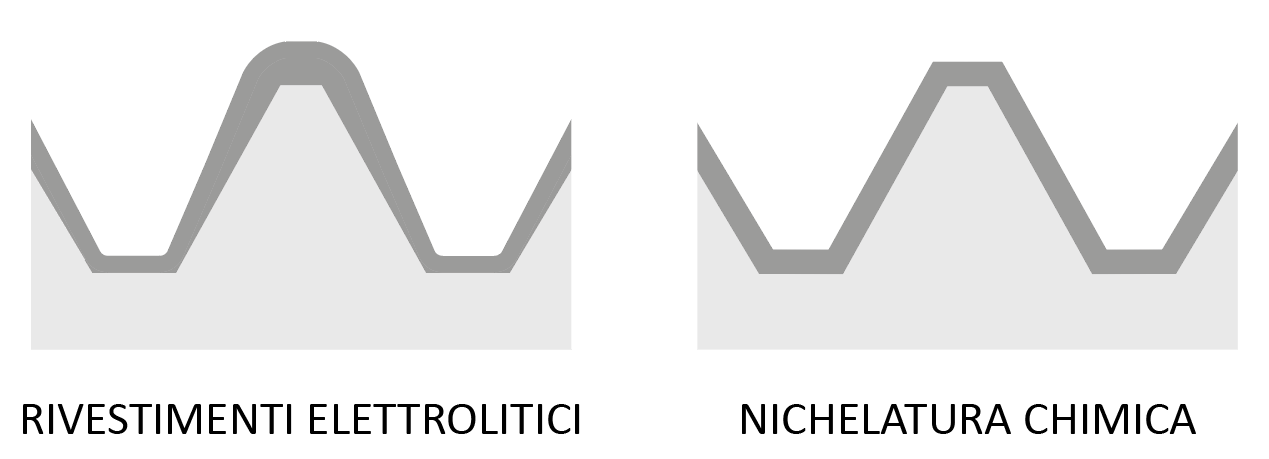
Electroless nickel and electrolytic nickel plating
Electroless nickel plating and electrolytic nickel plating can be said to have only the type of metal in common. The treatment processes are completely different, as are the surface characteristics and fields of application.
Electrolytic nickel plating is mainly, but not exclusively, used in the decorative field to provide corrosion resistance and a silvery metallic appearance. It is usually combined with an underlying copper coating to increase adhesion and to provide a bright, shiny appearance. It has a medium hardness and poor corrosion resistance.
Electroless nickel plating, on the other hand, is chosen in the technical field to increase hardness up to 1000 HV, increase wear resistance, reduce the coefficient of friction and give excellent corrosion resistance. It is applicable to all iron, aluminium, copper and titanium alloys.
Technical reference standards
The technical specifications and international standards for electroless nickel are as follows:
| Standard | Title |
|---|---|
| ISO 4527 | Metallic coatings - Autocatalytic (electroless) nickel-phosphorus alloy coatings - Specification and test methods |
| ASTM B733 | Standard Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal |
| MIL-C-26074 | Coatings, Electroless Nickel, Requirement for |
| AMS C26074 | Electroless Nickel Coatings |
| AMS 2404 | Plating, Electroless Nickel |
| AMS 2405 | Electroless Nickel Plating Low Phosphorus |
The most widely used and recognised standard is ISO 4527, which defines requirements and test methods for autocatalytic nickel-phosphorus alloy chemical coatings applied to metal substrates from aqueous solutions.
Types of electroless nickel
Coatings are distinguished according to the amount of phosphorus present in the alloy:
| % Phosphorus | Types of electroless nickel | Info |
|---|---|---|
| 1-4% P | Electroless Nickel low phosphorus | Not widely used industrially |
| 5-9% P | Electroless Nickel medium phosphorus Niplate 600 | Preferred for higher hardness and wear resistance and lower cost |
| >10% P | Electroless Nickel high phosphorus Niplate 500 | Preferred for higher corrosion resistance |
With the aim of increasing the surface properties of electroless nickel coatings, some coatings have been developed incorporating particles with particular properties, such as:
- Electroless nickel + PTFE: nanometric PTFE particles are co-deposited within the electroless nickel layer in a concentration of 25-35% by volume. Thanks to the properties of PTFE, the Niplate 500 PTFE coating has a very low coefficient of friction (0.08-0.10) with high corrosion resistance, good hardness and excellent adhesion to the base metal, a feature often lacking in sprayed PTFE coatings.
- Electroless nickel + SiC: for applications where wear resistance is the highest priority, the Niplate 600 SiC composite coating has been developed. Micrometric particles of silicon carbide (SiC), a ceramic material with high hardness, give the electroless nickel coating exceptional resistance to both abrasive and adhesive wear, superior to chrome plating.
Aesthetic appearance
Electroless nickel has a bright metallic appearance with a colour similar to stainless steel. It has a high resistance to oxidation and therefore maintains its colour and brilliance for a long time.
The surface morphology of the part and the roughness are not altered by the coating and therefore the final appearance will reflect the initial appearance.
A matt finish can be achieved using the processes of sandblasting, shot peening or shot blasting.
Electroless nickel + PTFE has a “gunmetal” grey colour.
Electroless nickel + SiC has a light grey colour.
Metals and alloys
Electroless nickel plating can be applied to most of the alloys commonly used in mechanical engineering:
- Carbon steel
- Stainless steel
- Case hardened, tempered and nitrided steels
- Aluminium alloys, extruded, rolled, cast and die-cast.
- Copper alloys, brass
- Titanium alloys
Coating thickness and tolerance
The thickness of the electroless nickel coating is uniform over the entire surface of the part.
Coating thicknesses between 5µm and 50µm are used, with a tolerance of ±10% and a minimum value of ±2µm. The standard thicknesses are as follows:
- 5±2µm
- 10±2µm
- 20±2µm
For certain severe wear or corrosion resistance applications, such as marine environments, thicknesses of 30±3 µm or 50±5 µm may be chosen, allowing the strength to be increased in proportion to the thickness.
The choice of the ideal thickness must be evaluated according to the requirements, the base material and the conditions of use, such as wear or the aggressiveness of the environment. Micron Srl is available to assist designers and mechanical workshops in the choice and definition of the correct thickness.
Hardness and heat treatments
The electroless nickel coating has a very high hardness, higher than almost any other metal alloy. Heat treatments carried out on electroless nickel coatings allow the hardness to be increased significantly, reaching a hardness of 1000 HV (69 HRC), exceeding the hardness of case hardened or nitrided steel. This significantly increases the wear resistance of the coated components, also thanks to the low friction coefficient of electroless nickel.
Embrittlement relief
During the electroless nickel plating process, atomic hydrogen diffusion occurs within the metal matrix, both in the coating and in the substrate. High-strength steels, due to this phenomenon, may exhibit so-called hydrogen embrittlement. During electroless nickel plating, since the deposition of the metal takes place without current, the hydrogen content that can diffuse into the metal is much lower than during traditional electroplated metal coatings such as chromium or electrolytic nickel plating. A heat treatment called embrittlement relief is usually carried out at 180°C for 4 hours to remove the hydrogen and improve the adhesion of the coating.
Hardening
The hardening heat treatments, which increase the hardness of the coating, are usually carried out at a temperature of 260-280°C for a hardness of about 800 HV, and 340°C for a hardness of about 1000 HV. Heat treatment at 260-280°C may cause a slight yellow colouring of the surface of the parts due to slight oxidation of the surface which occurs at those temperatures. The hardening treatment at 340°C is usually carried out in an air oven and causes an iridescent yellow-blue colouring on the surface of the pieces. The same hardening treatment can alternatively be carried out in a controlled atmosphere oven which allows the metallic appearance of electroless nickel to be maintained.
Corrosion resistance
Electroless nickel plating is a coating that provides excellent corrosion resistance. Corrosion behaviour depends strongly on the metal alloy coated, and for this reason, some clarifications should be made.
On iron alloys, the best corrosion resistance is obtained with the zinc treatment, as zinc is sacrificial and corrodes first, preventing corrosion of the iron. Electroless nickel is chosen instead of zinc plating when it is necessary to protect internal areas, maintain tight tolerances, have better adhesion and resistance to wear and scratches and when the piece is in contact with slightly acidic or alkaline substances.
On aluminium alloys, electroless nickel battles with anodising, differing in greater surface hardness, better chemical resistance in non-neutral environments, lower coefficient of friction and roughness, electrical conductivity and protection of all surfaces, including internal ones.
On copper alloys (brass), electroless nickel plating gives excellent corrosion resistance, being able to achieve up to 1000 hours of neutral salt spray NSS without any corrosion appearing.
Electroless nickel has excellent chemical resistance in contact with neutral or slightly aggressive environments, it oxidises with difficulty and preserves the metallic surface appearance. It resists well in contact with hydrocarbons, alcohols, neutral salt solutions, dilute reducing acids and dilute bases. It has difficulty in contact with concentrated acids and bases, especially oxidising ones.
Magnetism
Medium phosphorus electroless nickel is ferromagnetic: it has the ability to become magnetic under the action of an external magnetic field. High-phosphorus electroless nickel is not ferromagnetic, but becomes ferromagnetic when subjected to temperatures above 250°C. For this reason, if the absence of magnetism is required, it is necessary to choose Niplate 500 high phosphorus electroless nickel plating without hardening treatments.
Roughness
Electroless nickel plating does not alter the surface roughness from machining, so it is possible to keep the values almost unchanged.
Grinding
Some particular applications require grinding after electroless nickel coating, such as hydraulic shafts that require very tight tolerances and precise couplings.
Although electroless nickel is very hard, it can be ground quite easily. A hardening treatment at 800 HV or 1000 HV is even preferred as it improves machinability and chip removal.
Weldability
Niplate electroless nickel is easily weldable. It is widely used on copper busbar in order to avoid surface oxidation of copper and to allow excellent solderability of electronic components. Electroless nickel not only protects copper from corrosion, but also maintains the surface characteristics over time as it does not oxidise or corrode.
Reach e Rohs
Niplate electroless nickel plating coatings are REACH and RoHS compliant as there are no substances with restrictions of use above the maximum tolerated concentrations and no SVHC present in quantities above 0.1% by weight.
