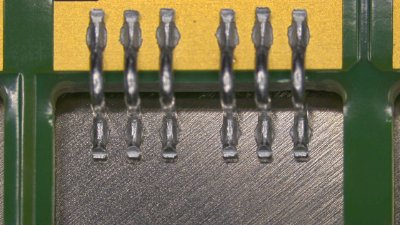
The term busbar refers to distribution bars used to transfer or distribute electrical energy, typically at high currents. At first glance they are simple components: a “clean” geometry, a defined cross-section, a conductive material. In practice—especially in e-mobility, inverters, battery packs, and power converters—the busbar becomes a critical element because connection performance depends not only on the volume of metal, but on the quality of the surface at the joint areas.
That’s where connection stability is decided: contact resistance, repeatability in bolted joints, behavior in brazing or soldering, and the ability to keep all of this consistent after storage and under real operating conditions (humidity, salt exposure, thermal cycling).
Copper and aluminum: two valid choices, the same challenge
Copper and aluminum are the two most widely used materials. Copper offers high conductivity and allows, at the same current level, more compact cross-sections and lower voltage drops. Aluminum provides clear advantages in weight and cost and, in many architectures, represents the best compromise when mass is a key design driver.
In both cases, however, there is a common factor that is often underestimated: the surface changes over time. Copper tends to oxidize and develop surface films that can make contact performance more variable. Aluminum quickly forms a tough, stable oxide layer—excellent as a natural protection of the metal, but challenging when a repeatable joint has to be achieved.
The outcome is simple: a busbar can be “perfect on the drawing,” yet become less predictable “in production” if the surface is not engineered to remain stable.
When the surface determines connection quality
In bolted connections, the key issue is contact resistance: oxide films or contamination can increase it and, above all, make it more dispersed from part to part. In soldered or brazed joints, on the other hand, the critical factor is wettability: if the surface is not clean and wettable, the process window for brazing narrows and the risk of defects rises (incomplete wetting, discontinuities, the need for more aggressive fluxes or more demanding parameters).
That is why surface finishing is not an aesthetic “nice-to-have” or a mere anti-corrosion coating: it becomes an integral part of connection design.
Why electroless nickel (Ni-P) is a robust technical choice
Electroless nickel plating deposits a Ni-P alloy with uniform thickness, without the build-up effects typical of some electrolytic processes, even on complex geometries.
This combination—uniformity, corrosion resistance, and surface stability—is what makes Ni-P particularly attractive when the goal is not only to protect, but to maintain more repeatable joint and contact performance over time.
Solderability that does not degrade during storage
Many electrical components are plated and then stored before assembly. If the surface evolves (oxidation, fingerprints, contamination), solderability can worsen: wettability decreases, variability increases, and joint quality becomes harder to control.
In this context, electroless nickel enables a functional surface engineered to preserve solderability over time.
NIPLATE® LINK: Ni-P designed for busbars and brazable components
For applications where the joint is the critical point, Micron developed NIPLATE® LINK
(patent application filed): a Ni-P electroless nickel plating specifically for electrical and connection components—such as copper busbars, connectors, and brazable parts—aimed at achieving a surface that remains solderable/brazable, corrosion-resistant, and stable over time.
From a metallurgical standpoint, the coating is a Ni-P alloy with a typical composition of Ni 85–90% and P 8–13%, selected to balance solderability and resistance to oxidation/corrosion. The intent is to reduce variability: a more consistent surface means more robust joining processes.
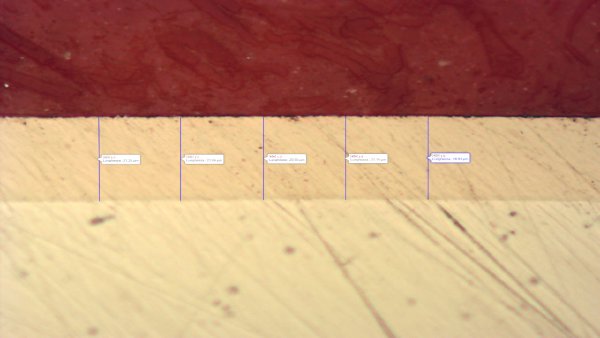
Typical thickness ranges from 5 to 15 µm with a tolerance of ±2 µm, with a light metallic, “stainless-like” bright appearance. For the normative framework of electroless nickel, common references include ISO 4527 and ASTM B733.
Corrosion and real environments: what changes in practice
In real power systems, beyond the joint itself, the environment matters: humidity, salt contamination, thermal cycling, possible condensation. Under these conditions, a well-designed Ni-P helps by limiting oxidation of the base metal and keeping the surface more stable.
NIPLATE® LINK, with thickness ≥ 5 µm on copper components, enables performance of > 1000 hours in neutral salt spray (NSS) per ISO 9227, with <1% corroded surface on a copper substrate (value referenced to test conditions and process qualification). In terms of chemical compatibility, the finish is generally suitable in the presence of humidity, saline environments, and many industrial fluids (hydrocarbons, technical oils, alcohols), while it is not suitable in the presence of oxidizing acids or concentrated bases.
This behavior is also of interest when the busbar operates near fluids or condensate, or when architectures include water-cooling circuits, because it helps protect the component from corrosion and prevents the release of substances and salts into the cooling fluid.
Where it makes the most sense: high-reliability power connections
Overall, NIPLATE® LINK is suitable when the connection must remain reliable over time and the joint is a critical process step: busbars for electric vehicles and HV distribution, contacts and terminals for battery packs and inverters (IGBT), copper components for power electronics and smart grids, and parts intended for soldering/brazing, including rail and renewable-energy applications.