aluminium anodizing, also known as anodic oxidation, is an electrolytic process by which a layer of aluminium oxide is formed on the surface of the workpiece to be treated, giving it excellent surface characteristics.
The most widely used aluminium anodizing process is carried out using a sulphuric acid solution as the electrolyte.
There is also an anodizing process called chromic anodizing, also known as Type I, which uses a chromic acid solution as the electrolyte. This coating is not widely used in industry because chromic acid is carcinogenic and is mainly used in the military and aeronautical sectors.
The parts to be treated are immersed in a tank containing the electrolyte, an anodic current is applied to the part (hence the name “anodizing”) and the cathodic current is applied to the sides of the tank. The sulphuric acid process is considered “eco-friendly” as no substances such as heavy metals, solvents or carcinogenic substances are used.
MAIN CHARACTERISTICS
Anodizing is the most used and most appreciated coating on aluminium as it gives excellent surface properties to the coated parts, at fairly low costs.
The main characteristics are:
- corrosion resistance
- wear resistance
- hardness
- thickness uniformity
- dielectric insulation
- possibility of coloring with dyes that penetrate into the oxide layer.
ANODIZABLE ALLOYS
Most of the aluminium alloys normally used in machining can be anodized easily and with excellent results.
The characteristics of the coating may differ depending on the alloy used, as it is a transformation process from aluminium to aluminium oxide and therefore the alloy composition may change its characteristics. The greatest difficulties are encountered with alloys that contain elements other than aluminium in high quantities, as it is only aluminium that contributes to forming the oxide layer. As a result, alloys containing high amounts of copper, such as the 2000 series, cannot achieve high thicknesses and the oxide layer will be slightly less compact and less resistant to corrosion and wear. Alloys containing more than 10% silicon may show color non-uniformity and may not achieve high thicknesses.
TYPES OF ANODIZING
Two different types of sulphuric acid anodizing can be distinguished, which differ both in their surface characteristics and in the process by which they are carried out:
- Type II or Clear anodizing, such as anodizing OX-A
- Type III or Hard anodizing, such as OX-HS and OX-W anodizing
The differences in the anodic layer and its surface characteristics are due to the use of different operating parameters such as the electrolyte temperature and the applied current (Volts and Amps). The equipment serving the anodizing process is also different for the two types.
TYPE II, CLEAR ANODIZING
Type II Clear anodizing, also called decorative anodizing, is mainly used for decorative or protective purposes in non-aggressive environments.
It is light grey in color and can be easily colored using dyes that penetrate into the oxide layer, thus guaranteeing excellent color rendering together with resistance to scratches and discoloration.
TYPE III HARD ANODIZING
Type III Hard anodizing improves and increases the characteristics of clear anodizing, thanks to a very dense and compact oxide layer with high hardness and excellent corrosion resistance. This treatment is mainly used in mechanical applications where excellent wear resistance is required, and in industrial or marine environments where aggressive agents are present.
STANDARDS
The main international standards related to anodizing are the following:
Clear anodizing
| Norma | Titolo |
|---|---|
| ISO 7599 | Anodizing of Aluminium and its alloys Method for specifying decorative and protective anodic oxidation coatings on Aluminium |
| UNI 10681 | Aluminium and aluminium alloys General characteristics of anodic oxide layers for use in decorative and protective field |
| MIL-PRF-8625, Type II | ANODIC COATINGS FOR ALUMINUM & ALUMINUM ALLOYS Type II: Sulfuric acid anodizing, conventional coatings produced from sulfuric acid bath |
Anodizzazione dura
| Norma | Titolo |
|---|---|
| ISO 10074 | Anodizing of Aluminium and its alloys Specification for hard anodic oxidation coatings on Aluminium and its alloys |
| UNI 7796 | Anodic oxidation coatings on aluminium and aluminium alloys Hard anodic films of great thickness - Requirements and general instructions of test |
| MIL-PRF-8625, Type III | ANODIC COATINGS FOR ALUMINUM & ALUMINUM ALLOYS Type III: Hard Anodic Coatings |
ANODIZING THICKNESS
| CLASS ISO 7599 | AVERAGE THICKNESS MINIMUM |
|---|---|
| AA 5 | 5 µm |
| AA 10 | 10 µm |
| AA 15 | 15 µm |
| AA 20 | 20 µm |
| AA 25 | 25 µm |
| SPECIFICATION | NOMINAL THICKNESS |
|---|---|
| ISO 10074 | 40 – 60 µm |
| MIL-PRF-8625 | ≃ 40 – 60 µm (2” ± 20%) |
| UNI 7796 | 30 – 60 µm |
DIMENSIONAL CALCULATION
In order to better understand the growth of anodizing thickness and correctly calculate dimensions, it is necessary to understand the mechanism of formation of the anodizing layer.
As we said at the beginning, anodizing is a process that converts aluminium into aluminium oxide forming a layer that can have various thicknesses.
The growth of aluminium oxide inevitably leads to a decrease in the metallic aluminium that is converted into oxide. It is therefore necessary to be careful not to consider the entire thickness of anodizing, but it is necessary to know how much in percentage the coating “penetrates” and how much it “grows”.
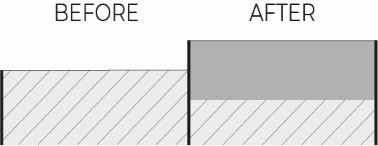
In precision mechanical engineering, the tolerances are very tight and the designer struggles to define them when coatings are applied, as the machining tolerances are added to those of the coating. It is therefore of fundamental importance that the final dimensions and tolerances of the coating are precisely and correctly defined in order to avoid errors, resulting in scrap or rework.
In clear anodizing applications, especially decorative ones, it is mistakenly assumed that anodizing does not create dimensional growth.
In such applications, tolerances are not critical and it is easier to assume that there is no growth.
In truth, in clear anodizing the oxide thickness creates a dimensional growth of about 30% of the anodizing thickness, which is usually 10µm, thus creating a growth of only 3µm.
A further variable that may slightly influence the final dimensions is the cleaning treatment prior to anodizing, called alkaline pickling, which slightly dissolves the surface, thus reducing the final dimension. This value cannot be defined in a standard way as it depends on the time spent in the tank and its concentration. It can range from a few microns in the case of precision mechanical components to tens of microns on extruded parts for which it is desired to remove extrusion lines.
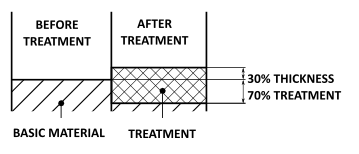
CLEAR ANODIZING
The coating grows 30% outward and 70% inward from the surface of the aluminium piece.
The <>radial dimensional increase is therefore equal to 30% of the treatment thickness.
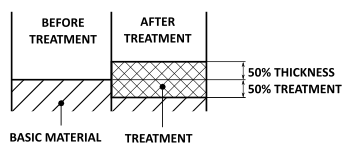
HARD ANODIZING
The treatment thickness increases 50% outward and 50% inward from the surface of the aluminium piece.
The radial dimensional increase is therefore equal to half the treatment thickness.
DIMENSION CALCULATOR
To assist designers and machine shops in setting dimensions and tolerances for coatings such as anodizing and electroless nickel plating, we have developed an automatic pre-treatment dimensional tolerance calculator. Simply enter the tolerances, the type of treatment and its thickness and the calculator automatically generates the pre-treatment machining tolerances to get the final toleranced dimensions correct.
